
You then calculate the integral of dq/T for this reversible path. Any reversible path between the two equilibrium states will suffice.
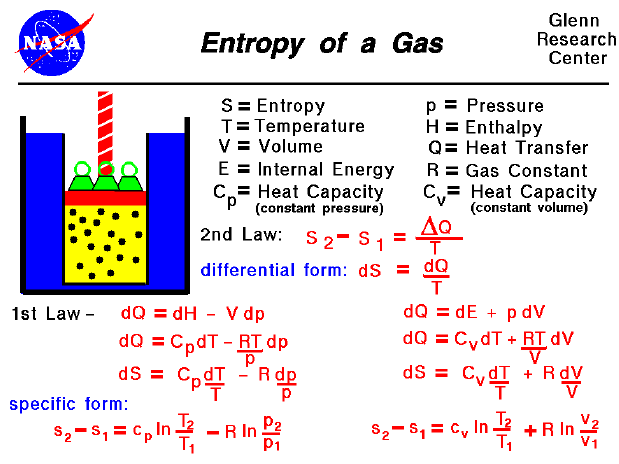
Then you need to conceive of a reversible path between the exact same initial and final equilibrium states (the reversible path may or may not necessarily bear any resemblance to the actual irreversible process). q res heat exchange for the system for reversible. The question is "how would we determine the change in entropy of a spontaneous process i.e irreversible process." To do this, you first focus exclusively on the initial and final thermodynamic equilibrium states of the system, resulting from the irreversible path. Randomness of any system is called entropy of system. TL DR: There IS no TL DR you're asking some important questions here, and the answers need to be made carefully. The Clausius form of the second law states that spontaneous change for an irreversible process in an isolated system (that is, one that does not exchange heat. For dQ of irreversible the equation should be changed into the clausius inequality form which is dS> dQ (irreversible)/T. Given the equation 2H2O + CO2 CH4 + 2O2, the entropies would be 188.7. The equation of dSdQ/T is not an accurate equation, the actual equation should be dSdQ (reversible)/T. Determine the standard entropies of all products and reactants using the entropy table.
#Change in entropy formula free
Small changes in intensive variables of the system are perfectly balanced by changes in those variables in the surroundings. One of the things which can be determined directly from this equation is the change in entropy during an isothermal expansion where N and U are constant. Irreversible free expansion of a gas in adiabatic condition is not isentropic.
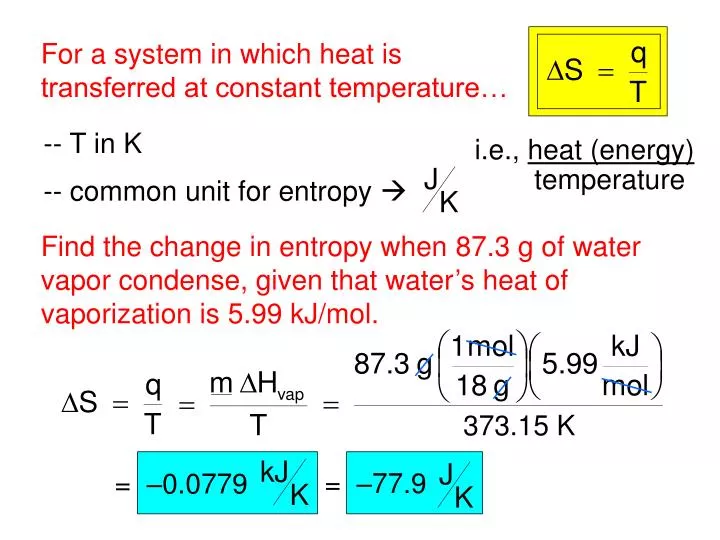
For freezing we calculate entropy change. Troutons rule estimates that it is 83-93 J mol-1K-1. Specifically, the system and its surroundings stay infinitesimally close to equilibrium with each other throughout a reversible process. Most liquids have nearly the same molar entropy of vaporization. A process is thermodynamically reversible if it is essentially at equilibrium.


 0 kommentar(er)
0 kommentar(er)
